Ammonia in freshwater and marine water
Toxicant default guideline values for protecting aquatic ecosystems
October 2000
Extracted from Section 8.3.7 ‘Detailed descriptions of chemicals’ of the ANZECC & ARMCANZ (2000) guidelines.
The default guideline values (previously known as ‘trigger values’) and associated information in this technical brief should be used in accordance with the detailed guidance provided in the Australian and New Zealand Guidelines for Fresh and Marine Water Quality.
Description of chemical
Ammonia (CAS 7664-41-7) is a basic industrial chemical, a soil nutrient and a common product of human and animal wastes. Other natural sources of ammonia are lightning, volcanic activity and decomposition of plant material.
The term ‘ammonia’ refers to two chemical species of ammonia that are in equilibrium in water: the un-ionised ammonia, NH3, and the ionised ammonium ion, NH4+. The proportion of the two chemical forms in water varies with the physico-chemical properties of the water, particularly pH and temperature. Ammonia is very soluble in water, the solubility being around 100,000 mg/L at 20°C. The concentrations of ammonia are usually expressed either as total ammonia (the sum of NH3 and NH4+) which takes into account the total amount as NH3 or N (Emerson et al. 1975), or as concentration of the un-ionised NH3 only. The concentrations can be given as component of N (e.g. NH3-N or total ammonia-N).
Uses and environmental fate of ammonia
Ammonia is a common industrial chemical for synthesis of many nitrogen-containing organic and inorganic chemicals, for manufacture of fertilisers or as a fertiliser itself by direct injection into soils, such as in irrigated cotton. The most common sources of ammonia entering surface waters and groundwaters are domestic sewage and industrial effluents.
Aquatic toxicology
Ammonia is a non-persistent and non-cumulative toxicant to aquatic life. The toxicity of ammonia can depend on pH, temperature and ionic composition of exposure water. The toxicity of ammonia is primarily attributed to the un-ionised NH3. Being a neutral molecule, un-ionised ammonia is able to cross epithelial membranes of aquatic organisms more readily than the ammonium ion. However, ammonium ion can also contribute significantly to ammonia toxicity under certain conditions.
In general, more un-ionised ammonia exists at higher pH and hence overall toxicity is greater, although the toxicity of the un-ionised form is less at higher pH. However, data also indicate that at lower pH, less un-ionised NH3 is needed to produce its toxic effects because the ammonium ion is responsible for some of the toxicity. At sufficiently lower pH, the relative amount of ammonium ion increases and it dominates toxicity. Overall, the effect of pH on toxicity of ammonia is largely explained by a combined toxicity of the un-ionised ammonia and ammonium ion, with un-ionised ammonia contributing mostly to toxicity at high pH and ammonium ion being more important at lower pH.
There are other effects of pH on the organism’s physiological and membrane processes that could alter ammonia toxicity, but these are not clearly established. In addition, the joint toxicity model cannot explain the temperature (Erickson 1985) and ionic composition effects. The effect of temperature on ammonia toxicity is not fully understood, although temperature in conjunction with pH, indirectly affect the speciation of ammonia in solution which in turn is the basis for the joint toxicity model for ammonia toxicity. The effect of ionic composition to ammonia toxicity is even much less understood even with recent data available (e.g. Iwama et al. 1997, Borgman & Borgman 1997).
There have been many reviews of ammonia toxicity (e.g. Alabaster & Lloyd 1982, Thurston & Russo 1983, USEPA 1985e, CCREM 1987). USEPA (1985e) found that ammonia was acutely toxic to freshwater organisms at concentrations (uncorrected for pH and temperature) ranging from 0.5 to 23 mg/L for nineteen invertebrate species and from 0.88 to 4.6 mg/L for 29 fish species. Invertebrates are generally more tolerant to ammonia than fish, and phytoplankton and aquatic vascular plants are more tolerant again (USEPA 1986, CCREM 1987). Salmonid fish appear to be particularly sensitive to ammonia. Acute toxicity to fishes may cause loss of equilibrium; hyperexcitability; increased breathing rate, cardiac output and oxygen uptake; and, in extreme cases, convulsions coma and death. Chronic effects of ammonia include a reduction in hatching success, reduction in growth rate and morphological development, and pathological changes in gill, liver and kidney tissue (USEPA 1986).
Factors that modify the toxicity of ammonia
The proportion of the total ammonia that is in the un-ionised form is highly dependent on pH and temperature (Emerson et al. 1975). Guidelines developed by USEPA (1986) have reflected the influence of these variations, with lower figures at higher temperatures and higher pH. At pH 8.5 and at 20°C, un-ionised ammonia contributes around 11% to the total ammonia concentration but at pH 6.0 at 20°C, it contributes only around 0.04% (CCREM 1987). Thurston et al. (1979) have developed equations for calculating the fraction of total ammonia that is un-ionised at pH values between 5 and 12 and temperatures between 0 and 40°C. Erickson (1985) has also produced useful equations for calculating un-ionised ammonia at different pH and temperature.
The following equations had been used in converting reported data on ammonia toxicity to total ammonia-N at the measured pH. The dissociation constant for ammonia at a given temperature is given by the relationship:
where T = 273.16 + t°C
The percentage of un-ionised ammonia at the reported pH is calculated using:
% un-ionised NH3 = 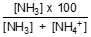
% un-ionised NH3 = 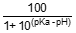
The figures obtained for percent un-ionised ammonia are reproduced in Table 8.3.6 for pH values between 6.5 and 8.5 and temperatures between 10°C and 30°C. If the concentration of ammonia is expressed as un-ionised ammonia, the total ammonia concentration can be calculated using the formula:
where [NH3] is concentration expressed as un-ionised ammonia.
Table 8.3.6 will be useful to allow water managers to calculate the concentration of un-ionised ammonia at given pH and temperature. Outside of these ranges, the equations above may be used. There are slight differences in the relationship between un-ionised ammonia, temperature and pH for marine and estuarine waters. Table 8.3.6 will give a reasonable approximation in these cases but managers may prefer to use the tables in Seager et al. (1988) or Bower and Bidwell (1978), which give figures at different salinity levels. The trigger values for ammonia (Table 8.3.7) have been derived from figures recalculated as total ammonia at a fixed pH (8.0).
Criteria developed overseas (e.g. USEPA 1986) have reflected the influence of variations of pH and temperature, with lower figures at higher temperatures and higher pH. Tables of overseas guidelines (USEPA 1986, CCREM 1987, Seager et al. 1988) indicate how guideline values change as the proportions of un-ionised ammonia change over ranges of pH between pH 6.5 and 9.0 and temperature between 0°C and 30°C. The USA tables have been recently revised (USEPA 1998), using a joint ammonia/ammonium ion concentration—response relationships for deriving acute and chronic values. They did not use non-US data but they omitted controversial data on white suckers. Although the increase in toxicity of total ammonia with increasing pH is well understood from the viewpoint of the ammonia-ammonium ion equilibrium (Erickson 1985), the pH dependence of un-ionised ammonia toxicity is less clear. Several authors have demonstrated some increase in toxicity of un-ionised ammonia with increasing pH to fish (Thurston et al. 1981, McCormick et al. 1984, Broderius et al. 1985) and invertebrates (Armstrong et al. 1978). In contrast, Tomasso et al. (1980) found that the toxicity of un-ionised ammonia did not vary significantly with pH in the range of 7–9. Erickson (1985) has plotted pH dependence of un-ionised ammonia toxicity demonstrated in these and other studies, as well as temperature dependence, using several different empirical models.
Other factors may modify the toxicity of ammonia, either by increasing the toxicity of un-ionised ammonia or by altering the concentration of un-ionised ammonia by a shift in the ammonia-ammonium ion equilibrium. These factors include dissolved oxygen, dissolved carbon dioxide, salinity, previous acclimation to ammonia, varying levels of exposure and the presence of other toxicants (USEPA 1985e, CCREM 1987).
Derivation of guideline trigger values
The USEPA (1998) recently revised its procedure for calculating acute and chronic ammonia criteria. Their general approach was adopted in deriving the present trigger values. The mathematical model for the derived values is based on a pH-dependent joint toxicity of un-ionised ammonia and ammonium ion, showing the importance of the role of speciation in ammonia toxicity. The effects of temperature and ionic composition are not sufficiently large or consistent enough to allow adjustment of the joint toxicity model, hence no temperature conversions were used in the procedure.
The guideline trigger values were derived from all acceptable data from literature, regardless of pH and temperature conditions. Various expressions of acutely toxic concentrations (EC50s) were converted to a uniform expression as total ammonia-N from the reported pH and temperature (see formulas above). Chronic data (EC20s) given in USEPA (1998) were included in the chronic dataset, along with NOEC values obtained from recent Australian and New Zealand studies. All chronic concentrations were expressed as total ammonia-N.
When acute (AV) and chronic (CV) concentrations were available, all values were converted to a common pH of 8 (AVpH8 and CVpH8 respectively) using the following formulas adopted from USEPA (1998):
For each species, the geometric mean of available AV pH8 and/or CV pH8 data were calculated to obtain mean acute and/or chronic value at pH 8. These mean acute and chronic values were evaluated for use in deriving the guideline trigger value. Since there was enough mean chronic data from 5 different species covering four taxonomic groups to meet the requirements (ANZECC & ARMCANZ 2000 Section 8.3.4.4) of deriving a high reliability trigger value, only the chronic values were eventually used. A freshwater trigger value of 900 µg/L total ammonia-N at the specific pH of 8 was obtained. Guideline trigger values at other pH conditions were then calculated using the same equations given above, and these are given in Table 8.3.7. The range of data used (USEPA 1998) in deriving the equations above indicates that they are applicable from pH of 6 to 9, although some error might exist at the lower end of the range for some species. Extrapolation at pH outside of 6 to 9 is not advisable, due to the lack of knowledge on the effects of ammonia at these extreme pH levels.
Aquatic toxicology
The values given below are geometric means of species data taken from all screened data that concurrently measured pH and temperature. Figures were adjusted to a standard pH of 8.0 and calculated in terms of total ammonia-N.
Freshwater fish: 15 species, 24 to 96-hour LC50, 3944-169,873 µg/L (an anomalous figure of 72 µg/L was extracted from AQUIRE [1994]). Chronic NOEC and EC20 for nine species (28-6 d growth and survival) of 1350 to 19,720 µg/L.
Freshwater crustaceans: 10 species, 24 to 96-hour LC50, 7754 to 108,500 µg/L. The cladoceran Simocephalus vetulus was the most sensitive (24-hour EC and LC50 values around 1580 µg/L) and the amphipod Crangonyx pseudogracilis was least sensitive. Chronic NOEC and EC20 for four species (7 days to 10 weeks, reproduction) of 1450 to 19,770 µg/L.
Freshwater insects: eight species, 24 to 96-hour LC50, 15091 to 282,400 µg/L. Chronic NOEC for two species (29 d, reproduction) of 1790 to 4400 µg/L.
Freshwater molluscs: 7 spp, 12,558 to 74,623 µg/L. Chronic NOEC and EC20 for two species (42 to 60 days, reproduction and survival) of 540 to 2620 µg/L. The most sensitive species under chronic exposure was the New Zealand species Sphaerium novaezelandiae with NOEC (60 days mortality and reproduction) of 540 µg/L total ammonia-N.
Freshwater annelid: two species, 24 to 96-hour LC50, 20,071 to 79,788 µg/L.
Freshwater rotifer: Brachionus rubens, 24-hour LC50 of 1300 µg/L.
Freshwater Platyhelminthes: Polycelus tenuis, 24 to 96-hour LC50 of 37,634 µg/L.
Marine fish: three species, 44-68 h LC50, 8800 (Pagrus major); 21,400 (Salmo salar) and 44,900 µg/L (Fundulus heteroclitus). NOEC figures (20 days) for sea bream Sparus auratus, were 6330 µg/L (mortality) and 3640 µg/L (growth).
Marine crustaceans: 15 species, 24 to 96-hour LC50, 18,687 µg/L (Penaeus semisulcatus) to 264,000 µg/L (brine shrimp Artemia salina); 11 species had LC50 values below 80,000 µg/L.
Marine molluscs: two species, 48 to 96-hour LC50, 7720 µg/L (Argopecten irradians) to 42,800 µg/L (Anadara granosa).
Marine rotifer: Brachionus plicatus, 24 to 96-hour EC50 population growth 101,000 µg/L.
The marine trigger values (moderate reliability) were derived from acute data and the freshwater trigger values (high reliability) from chronic data.
Australian and New Zealand data
The data given below refer to values that have not been converted to common pH of 8.
Hickey and Martin (1999) reported that the New Zealand freshwater fingernail clam Sphaerium novaezelandiae was very sensitive to ammonia in 60-day exposures at pH 7.5 and 20°C. LC50 and IC50 (juvenile production) figures respectively were 37 and 13 µg/L, based on un-ionised ammonia (NH3-N), and 3800 and 800 µg/L based on total ammonia (N). These are found commonly in lowland streams in New Zealand and similar species may also occur in Australia. These are among the most sensitive figures and may need consideration in site-specific assessments. If users consider that it is important to protect these or related clams at the site, either the 95% trigger value could be divided by a factor of 2 or the 99% protection level adopted at the specific site. As this was the only species from a large dataset with figures below the trigger value, the 95% protection level was considered appropriate for most slightly to moderately disturbed ecosystems.
Hickey and Vickers (1994) reported acute toxicity values for nine New Zealand species at 15°C and pH 7.6 or 8.2: crustaceans Paracalliope fluviatili (amphipod); Paratya curvirostris (shrimp), insects Deleatidium spp, Pycnocentria evecta, Zephlebia dentata, Zealandobius furcillatus; annelid Lumbriculus variegatus; molluscs Potamopyrgus antipodarum (snail), Sphaerium novaezelandiae (fingernail clam). They reported that temperature had no significant effect on toxicity of un-ionised ammonia to snails tested at 15, 20 and 25°C.
Hickey et al. (1999) used freshwater stream mesocosms at 16°C to determine chronic toxicity (29 d) of ammonia to New Zealand macroinvertebrate communities. Only two mayfly species showed significant decreases in abundance at the concentrations tested: the 29-day EC50 values for total and un-ionised ammonia for Deleatidium sp. were 2.15 mg N/L and 0.145 mg N/L respectively; the NOECs were 950 and 66 g/L respectively. NOECs for Coloburiscus humeralis were 2330 and 160 g/L respectively.
Richardson (1991) determined 96-h LC50 for juvenile inanga (Galaxias maculatus) at 15°C pH 8.2 was 1600 µg NH3/L un-ionised ammonia.
Richardson (1997) determined acute toxicity of ammonia to seven New Zealand indigenous fish (banded kokopu Galaxias fasciatus, common bully Gobiomorphus cotidianus, common smelt Retropinna retropinna, redfin bully G. huttoni, inanga Galaxias maculatus, and longfin and shortfin eels Anguilla dieffenbachii and A. australis; and one indigenous crustacean species. Shrimp (Paratya curvirostris) was the most sensitive. The 9-hour LC50 at 15°C pH 7.5 or 8.2 ranged from 0.75 to 2.35 mg/L NH3/L for these species.
Guidelines
Guideline trigger values were calculated by converting all acceptable chronic NOEC data, reported at different pH values, to total ammonia at a common pH value of 8 before applying the statistical distribution derivation method. No temperature conversions were used in the procedures. Water managers need to refer to Table 8.3.7 in the section on ammonia every time that ammonia toxicity is being considered. It is important to determine the pH and temperature whenever ammonia concentrations are measured. When ammonia concentration is expressed as that of un-ionised ammonia instead of total ammonia, Table 8.3.6 can be used to derive total ammonia. Table 8.3.6 reports the percentage of un-ionised to total ammonia at different pH and temperatures.
A freshwater high reliability trigger value of 900 µg/L TOTAL ammonia-N was calculated at pH 8.0 using the statistical distribution method with 95% protection. This translates to about 35 µg/L un-ionised ammonia-N at 20⁰C. Table 8.3.7 of ANZECC & ARMCANZ (2000) indicates how the guideline figure changes at different pH values.
Table 8.3.6 Percentage of un-ionised ammonia at different pH and temperatures; from ANZECC & ARMCANZ (2000)
| pH | 6.5 | 6.6 | 6.7 | 6.8 | 6.9 | 7.0 | 7.1 | 7.2 | 7.3 | 7.4 | 7.5 | 7.6 | 7.7 | 7.8 | 7.9 | 8.0 | 8.1 | 8.2 | 8.3 | 8.4 | 8.5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10oC | 0.0588 | 0.0740 | 0.0931 | 0.117 | 0.148 | 0.186 | 0.234 | 0.294 | 0.370 | 0.465 | 0.585 | 0.735 | 0.924 | 1.16 | 1.46 | 1.83 | 2.29 | 2.86 | 3.58 | 4.46 | 5.56 |
| 12.5oC | 0.0714 | 0.0899 | 0.113 | 0.142 | 0.179 | 0.225 | 0.284 | 0.357 | 0.449 | 0.564 | 0.710 | 0.892 | 1.12 | 1.41 | 1.76 | 2.21 | 2.77 | 3.46 | 4.31 | 5.37 | 6.67 |
| 15oC | 0.0865 | 0.109 | 0.137 | 0.172 | 0.217 | 0.273 | 0.343 | 0.432 | 0.543 | 0.683 | 0.858 | 1.08 | 1.35 | 1.70 | 2.13 | 2.66 | 3.33 | 4.16 | 5.18 | 6.43 | 7.96 |
| 17.5oC | 0.104 | 0.131 | 0.165 | 0.208 | 0.262 | 0.329 | 0.414 | 0.521 | 0.655 | 0.823 | 1.03 | 1.30 | 1.63 | 2.04 | 2.56 | 3.20 | 3.99 | 4.97 | 6.18 | 7.66 | 9.45 |
| 20oC | 0.125 | 0.158 | 0.199 | 0.250 | 0.315 | 0.396 | 0.498 | 0.626 | 0.786 | 0.988 | 1.24 | 1.56 | 1.95 | 2.45 | 3.06 | 3.82 | 4.76 | 5.92 | 7.34 | 9.07 | 11.2 |
| 22.5oC | 0.150 | 0.199 | 0.238 | 0.300 | 0.377 | 0.474 | 0.596 | 0.749 | 0.942 | 1.18 | 1.48 | 1.86 | 2.33 | 2.92 | 3.65 | 4.55 | 5.66 | 7.02 | 8.68 | 10.7 | 13.1 |
| 25oC | 0.180 | 0.226 | 0.285 | 0.358 | 0.450 | 0.566 | 0.712 | 0.895 | 1.12 | 1.41 | 1.77 | 2.22 | 2.78 | 3.47 | 4.33 | 5.39 | 6.69 | 8.28 | 10.2 | 12.5 | 15.3 |
| 27.5oC | 0.214 | 0.270 | 0.339 | 0.427 | 0.536 | 0.674 | 0.848 | 1.06 | 1.34 | 1.68 | 2.10 | 2.63 | 3.29 | 4.11 | 5.12 | 6.36 | 7.87 | 9.72 | 11.9 | 14.6 | 17.7 |
| 30oC | 0.255 | 0.320 | 0.403 | 0.507 | 0.637 | 0.801 | 1.01 | 1.26 | 1.58 | 1.99 | 2.49 | 3.11 | 3.89 | 4.85 | 6.02 | 7.47 | 9.22 | 11.3 | 13.9 | 16.9 | 20.3 |
Illustration on how to calculate concentration of total ammonia-N (as µg/L): for a solution with un-ionised NH3 of 68.7 µg/L at pH 7 and temperature 25°C, the concentration of total ammonia-N is: total ammonia-N (µg/L) = un-ionised ammonia as µg NH3/L x (14/17) / (% un-ionised ammonia/100); 68.7 µg/L as NH3 x (14/17) / (0.566/100) = 10 000 µg/L total ammonia-N.
Table 8.3.7 Freshwater trigger values as total ammonia-N in µg/L at different pH (temperature is not taken into consideration); from ANZECC & ARMCANZ (2000)
| pH | Freshwater trigger value (µg/L as total ammonia-N) |
Marine trigger value (µg/L as total ammonia-N) |
|---|---|---|
| 6.0 | 2570 | 5960 |
| 6.1 | 2555 | 5870 |
| 6.2 | 2540 | 5760 |
| 6.3 | 2520 | 5630 |
| 6.4 | 2490 | 5470 |
| 6.5 | 2460 | 5290 |
| 6.6 | 2430 | 5070 |
| 6.7 | 2380 | 4830 |
| 6.8 | 2330 | 4550 |
| 6.9 | 2260 | 4240 |
| 7.0 | 2180 | 3910 |
| 7.1 | 2090 | 3560 |
| 7.2 | 1990 | 3200 |
| 7.3 | 1880 | 2840 |
| 7.4 | 1750 | 2490 |
| 7.5 | 1610 | 2150 |
| 7.6 | 1470 | 1850 |
| 7.7 | 1320 | 1560 |
| 7.8 | 1180 | 1320 |
| 7.9 | 1030 | 1100 |
| 8.0 | 900 | 910 |
| 8.1 | 780 | 750 |
| 8.2 | 660 | 620 |
| 8.3 | 560 | 510 |
| 8.4 | 480 | 420 |
| 8.5 | 400 | 350 |
| 8.6 | 340 | 290 |
| 8.7 | 290 | 240 |
| 8.8 | 240 | 200 |
| 8.9 | 210 | 170 |
| 9.0 | 180 | 140 |
The 95% figure is considered sufficiently protective of most slightly to moderately disturbed systems. However, this figure may not be sufficiently protective of the freshwater clam Sphaerium novazelandiae and related species. If these are significant at a site, site-specific studies or a higher protection level may be warranted. See notes under Australian and New Zealand data on appropriate site-specific approaches.
A marine moderate reliability trigger value of 910 µg/L TOTAL ammonia-N was calculated at pH 8.0 statistical distribution method with 95% protection. Table 8.3.7 indicates how the guideline figure changes at different pH values.
The differences in the degree to which the marine and freshwater trigger values change with pH are due to the different data types and the different equations applicable to each system.
References
Alabaster JS & Lloyd R 1982. Water quality criteria for freshwater fish. Butterworth Scientific, London.
ANZECC & ARMCANZ 2000. Australian and New Zealand Guidelines for Fresh and Marine Water Quality, Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra.
Armstrong DA, Chippendale D, Knight AW & Colt JE 1978. Interaction of ionised and unionised ammonia on short-term survival and growth of prawn larvae, Macrobrachium rosenbergii. Biological Bulletin 154, 15-31.
Bower CE & Bidwell JP 1978. Ionization of ammonia in seawater: Effects of temperature, pH and salinity. Journal of the Fisheries Research Board Canada 35, 1012-1016.
Broderius SJ, Drummond RA, Fiandt JT & Russom CL 1985. Toxicity of ammonia to early life stages of the smallmouth bass at four pH values. Environmental Toxicology and Chemistry 4, 87-96.
CCREM 1987. Canadian water quality guidelines. Canadian Council of Resource and Environment Ministers, Ontario.
Emerson K, Russo RC, Lund RE & Thurston RV 1975. Aqueous ammonia equilibrium calculations: Effect of pH and temperature. Journal of the Fisheries Research Board of Canada 32, 2379-2383.
Erickson RJ 1985. An evaluation of mathematical models for the effects of pH and temperature on ammonia toxicity to aquatic organisms. Water Research 19, 1047–1058.
Hickey CW, Golding LA, Martin M L & Croker GF 1999. Chronic toxicity of ammonia to New Zealand freshwater invertebrates: A mesocosm study. Archives of Environmental Contamination and Toxicology 37, 338–351.
Hickey CW & Martin ML 1999. Chronic toxicity of ammonia to the freshwater bivalve Sphaerium novaezelandiae. Archives of Environmental Contamination and Toxicology 36, 38–46.
Hickey CW & Vickers ML 1994. Toxicity of ammonia to nine native New Zealand freshwater invertebrate species. Archives of Environmental Contamination and Toxicology 26, 292–298.
Iwama GK, McGeer JC, Wright PA, Wilkie MP & Wood CM 1997. Divalent cations enhance ammonia excrection in Lahotan cutthroat trout in highly alkaline water. Journal of Fish Biology 50, 1061–1073.
McCormick JH, Broderius SJ & Fiandt JT 1984. Toxicity of ammonia to early life stages of the green sunfish (Lepomis cyanellus). Environmental Pollution (Series A) 34, 147-163.
Richardson J 1991. Acute toxicity of ammonia to juvenile inanga (Galaxias maculatus). New Zealand Journal of Marine and Freshwater Research 25, 327–330.
Richardson J 1997. Acute ammonia toxicity for eight New Zealand indigenous freshwater species. New Zealand Journal of Marine and Freshwater Research 31, 185–190.
Seager J, Wolff EW & Cooper VA 1988. Proposed environmental quality standards for list II substances in water: Ammonia. ESSL TR 260, Water Research Centre, Medmenham, UK.
Thurston RV & Russo RC 1983. Acute toxicity of ammonia to rainbow trout. Transactions of the American Fisheries Society 112, 696-704.
Tomasso JR, Goudie CA, Simco BA & Davis KB 1980. Effects of environmental pH and calcium on ammonia toxicity in channel catfish. Transactions of the American Fish Society 109, 229-234.
USEPA 1985e. Ambient water quality criteria for ammonia — 1984. Criteria and Standards Division, US Environmental Protection Agency, Washington DC. EPA-441/5-85-001.
USEPA 1986. Quality criteria for water. US Department of Commerce, National Technical Information Service, US Environmental Protection Agency, Springfield, Virginia. PB87-226759, EPA 440/5 86-001.
USEPA 1998. Update of ambient water quality criteria for ammonia. US Environmental Protection Agency, Washington DC, EPA 822-R-98-008.










